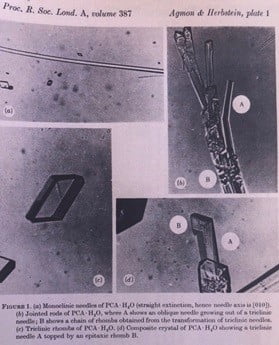
This photomicrograph on the left in water shows the needle physical shapes of PCA in early phase of going into solution. Note the prism shapes not yet converted to needle shapes in interval of 30 minutes. On the right the PCA is already needle shaped in alcohol solution in the same time frame due to increased solubility in alcohol as compared to water.
The associated in vitro studies reported below replicated the clinical environment in reducing transmission. The PCA crystal coating on a hard or cloth article was viricidal for SARS CoV2 virus. The same would be for any coating on skin or other like surfaces.
Low pH: Drugs that change the pH at the surface of the cell membrane and inhibit the fusion of the virus to the cell membrane. It can also inhibit nucleic acid replication, glycosylation of viral proteins, virus assembly, new virus particle transport, virus release, and other processes to achieve its antiviral effects.
Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug.
Semin Arthritis Rheum 1993; 23:82–91.
PCA has an acid pH of 5.4 which is disruptive to viral coating.
Takeda Y1, Okuyama Y2, Nakano H3, Yaoita Y2, Machida K2, Ogawa H4, Imai K5.
Antiviral Activities of Hibiscus sabdariffa L. Tea Extract Against Human Influenza A Virus Rely Largely on Acidic pH but Partially on a Low-pH-Independent Mechanism. Food Environ Virol. 2020 Mar;12(1):9-19. doi: 10.1007/s12560-019-09408-x. Epub 2019 Oct 16.
Abstract: Influenza A virus (IAV) infection is perennially one of the leading causes of death worldwide. Effective therapy and vaccination are needed to control viral expansion. However, current anti-IAV drugs risk inducing drug-resistant virus emergence. Although intranasal administration of whole inactivated virus vaccine can induce efficient protective immunity, formalin and β-propiolactone are the currently used and harmful inactivating agents. Here, we analyzed the antiviral activity of hibiscus (Hibiscus sabdariffa L.) tea extract against human IAV and evaluated its potential as a novel anti-IAV drug and a safe inactivating agent for whole inactivated vaccine. The in vitro study revealed that the pH of hibiscus tea extract is acidic, and its rapid and potent antiviral activity relied largely on the acidic pH. Furthermore, the mouse study showed that the acidic extract was not effective for either therapeutic or vaccination purposes. However, hibiscus tea extract and protocatechuic acid, one of the major components of the extract, showed not only potent acid-dependent antiviral activity but also weak low-pH-independent activity. The low-pH-independent activity did not affect the conformation of immunodominant hemagglutinin protein. Although this low-pH-independent activity is very limited, it may be suitable for the application to medication and vaccination because this activity is not affected by the neutral blood environment and does not lose antigenicity of hemagglutinin. Further study of the low-pH-independent antiviral mechanism and attempts to enhance the antiviral activity may establish a novel anti-IAV therapy and vaccination strategy.
The pH based (3.6) has Selective Effect on Various Food Borne Virus.
Joshi SS, Dice L, D’Souza DH. Aqueous Extracts of Hibiscus sabdariffa Calyces Decrease Hepatitis A Virus and Human Norovirus Surrogate Titers. Food Environ Virol. 2015 Dec;7(4):366-73. doi: 10.1007/s12560-015-9209-1. Epub 2015 Jul 5.
Abstract: Hibiscus sabdariffa extract is known to have antioxidant, anti-diabetic, and antimicrobial properties. However, their effects against foodborne viruses are currently unknown. The objective of this study was to determine the antiviral effects of aqueous extracts of H. sabdariffa against human norovirus surrogates (feline calicivirus (FCV-F9) and murine norovirus (MNV-1)) and hepatitis A virus (HAV) at 37 °C over 24 h. Individual viruses (~5 log PFU/ml) were incubated with 40 or 100 mg/ml of aqueous hibiscus extract (HE; pH 3.6), protocatechuic acid (PCA; 3 or 6 mg/ml, pH 3.6), ferulic acid (FA; 0.5 or 1 mg/ml; pH 4.0), malic acid (10 mM; pH 3.0), or phosphate buffered saline (pH 7.2 as control) at 37 °C over 24 h. Each treatment was replicated thrice and plaque assayed in duplicate. FCV-F9 titers were reduced to undetectable levels after 15 min with both 40 and 100 mg/ml HE. MNV-1 was reduced by 1.77 ± 0.10 and 1.88 ± 0.12 log PFU/ml after 6 h with 40 and 100 mg/ml HE, respectively, and to undetectable levels after 24 h by both concentrations. HAV was reduced to undetectable levels by both HE concentrations after 24 h. PCA at 3 mg/ml reduced FCV-F9 titers to undetectable levels after 6 h, MNV-1 by 0.53 ± 0.01 log PFU/ml after 6 h, and caused no significant change in HAV titers. FA reduced FCV-F9 to undetectable levels after 3 h and MNV-1 and HAV after 24 h. Transmission electron microscopy showed no conclusive results. The findings suggest that H. sabdariffa extracts have potential to prevent foodborne viral transmission.
Anti-Protease: COVID-19 main protease (Mpro) is the key enzyme of coronavirus which plays crucial role in virus replication and transcription, which can be targeted to retard the growth of virus inside the host.
Bhatia S, Giri S, Lal AF, Singh S. Battle Against Coronavirus: Repurposing Old Friends (Food Borne Polyphenols) for New Enemy (COVID-19). ChemRxiv. 2020. https://doi.org/10.26434/chemrxiv.12108546.v1
https://chemrxiv.org/articles/Battle_Against_Coronavirus_Repurposing_Old_Friends_Food_Borne_Polyphenols_for_New_Enemy_COVID-19_/12108546/1
One of the major protein of COVID 19 is Mpro (main protease), also referred to as the “3C-like protease” belonging to the proteases class of hydrolytic enzymes. This enzyme plays a key role in the processing of pp1a (responsible for generating copies of viral genome) and pp1ab (responsible for generating viral genome) as involved in their proteolytic cleavage at the conserved residues among COVID 19 genome.
These can assemble to give rise to virions inside the host cell and thus, replicate to produce multiple copies as shown in Figure 2. Mpro can act as potential target for structure based drug discovery as this enzyme not only involved in autocatalytic cleavage of itself and key viral enzymes, as well as lacks any close homologues among human host. Targeting this enzyme using suitable protease small molecule inhibitor holds immense potential to curb virus replication and transcription which are critical steps in virus life cycle.
Chandani SR, Lokhande KB, Swamy KV, Nanda RK, Chitlange SS. Data on docking of phytoconstituents of Actinidia deliciosa on dengue viral targets. Data Brief. 2019;25:103996. Published 2019 May 17. doi:10.1016/j.dib.2019.103996
Anti-docking: Molecular docking is an important tool in computer-based drug design and drug discovery which helps to predict the small ligand conformation and orientation (Docking pose) within the active sites of the target receptor protein.
Protocatechuic acid has high docking score (-9.8) and importantly protocatechuic acid derivatives shows comparatively better pharmacokinetic predictions and lead likeness, along with the ease of synthesis. PCA is bioavailable by oral intake as in black tea the focus of this report.
PCA as one of the polyphenolic scaffolds have affinity to bind with substrate-binding pocket of COVID-19 virus Mpro ,which is highly conserved among all CoV Mpros. This investigation intensely supports our hypothesis that small molecule inhibitors (PCA) targeting Mpro or in combination with other adjuvant therapies could provide an effective therapeutic regime to fight against all coronavirus associated diseases.
The top six docked polyphenols which were mainly derivatives of protocatechuic acid polyphenols.
Their pharmacokinetics study pointed out the poor bioavailability of these polyphenols if taken 22 individually as active compound. However, protocatechuic acid (1 Lipinski violation) derivative among all have shown better pharmacokinetic profile.
The focus of this study suggested that the dietary intake of “black tea” can improve the resistance to fight against COVID 19 virus in early stages of human infection. Importantly though, the enriched subset of six compounds identified from the larger library has to be validated experimentally. PCA is one of the ingredients needing further study.
Ganesh SM, Awasthi P, Timiri AK, Ghosh M. A novel approach for rationale selection of medicinal plants against viruses via molecular docking studies. December 2014. In
Oak Jubilee Volume 2015; pages 18-30. ISSN 0379-556X (Print)/ ISSN 2347-6001 (Online) The Pharmstudent. https://old.iitbhu.ac.in/phe/pharmsociety/issue_2015/3.GaneshM_et_al.pdf
In Ganesh we learn that flavonoids are found to be good antiviral agents and have excellent docking scores and inhibition constant values. PCA is a flavonoid and found in Euphorbia hirta which is rich in flavonoids; i.e. protocatechuic acid. PCA has anti-protease inhibition.
The following publication showed PCA docking potential in HIV-1 virus.
Pattarapan Panthong, Kingkan Bunluepuech, Nawong Boonnak, Prapaporn Chaniad, Somsak Pianwanit, Chatchai Wattanapiromsakul & Supinya Tewtrakul (2015) Anti-HIV-1 integrase activity and molecular docking of compounds from Albizia procera bark, Pharmaceutical Biology, 53:12, 1861-1866, DOI: 10.3109/13880209.2015.1014568
Enhanced Hormonal and cellular immunity: The results of Ou, et al indicate that PCA may improve the poultry health by enhancing both the humoral and cellular immune response.
Guo Y, Zhang Q, Zuo Z, et al. Protocatechuic acid (PCA) induced a better antiviral effect by immune enhancement in SPF chickens. Microb Pathog. 2018;114:233-238. doi:10.1016/j.micpath.2017.11.068
This evidence is particularly important in that recent reports have shown COVID19 has changed form a biological disease to one of attacking the patient’s immunity.
Anti-inflammatory properties as a catabolic cytokine blocker: One of the ways the human body responds to SARS CoV-2 is with a massive anti-inflammatory response. This has been termed a “cytokine storm” in the lungs. It results in further inflow of fluids to the already compromised lungs.
PCA has powerful anti-inflammatory properties with clinical relevance for the “cytokine storm” associated with clinical SARS CoV2 infections. It also reduced the C-reactive protein (Lin, et al)
Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem. 2009;57(15):6661-6667. doi:10.1021/jf9015202
Lende AB, Kshirsagar AD, Deshpande AD, et al. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacol.12 July 2011. DOI 10.1007/s10787-011-0086-4
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/fulltext
PCA has powerful anti-inflammatory by its anti-catabolic cytokine blocker properties.
Anti-tyrosinase Inhibitor: PCA acts as a tyrosinase inhibitor in other applications and has potential in viral treatment.
Miyazawa M, Oshima T, Koshio K, Itsuzaki Y, Anzai J. Tyrosinase Inhibitor from Black Rice Bran. J. Agric. Food Chem. 2003, 51, 24, 6953-6956. October 28, 2003.
https://doi.org/10.1021/jf030388s
Van Tieghem N, Doyen A, Liteanu D, Cogniaux J, Frühling J. Modulation of tyrosinase activity and viral information by 5-iodo-deoxyuridine and L-dopa in a human melanoma cell line [proceedings]. Arch Int Physiol Biochim. 1979;87(4):857-858.
Anti-thrombosis Properties: PCA, similar in structure to aspirin will slightly increase the prothrombin time. This is especially beneficial for prophylactic or therapeutic treatment in covid19 clinical cases that may have thrombosis.
Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, anti-inflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem. 2009;57(15):6661-6667. doi:10.1021/jf9015202.
Huang L, Lin C, Li A, Wei B, Teng J, Li L. Pro-coagulant activity of phenolic acids isolated from Blumea riparia. Nat Prod Commun. 2010;5(8):1263-1266.
Independent Contract Laboratory Testing: The experimental model was such that it primarily replicated the clinical liquid environment in which the SARS CoV-2 comes in contact with the crystals of PCA. This method replicated the transmission and the environment for treatment.
A dry environment was created at 60 minutes in the first phase of one of the labs testing, so both liquid and drying of the crystal coating was tested.
Illinois Technical Institute
July 17, 2020
Dr. Lanny L. Johnson, M.D.
314 East Crystal Downs Drive
Frankfort, MI 49635
Re: Study 2969001001001: Testing Protocatechuic Acid (PCA) as a Possible Treatment Against SARS-CoV-2 Infected cells in vitro
Dear Dr. Johnson,
The study investigation (Study # 2969001001001) of the effectiveness of Protocatechuic Acid (PCA) against SARS-CoV-2, the causative virus for COVID19 has been completed. Testing of the PCA was initiated on June 16, 2020.
Test Article and Test Substrate identification and preparation.
The Test Article (TA) used for this study was Protocatechuic Acid (PCA) and was provided by the Sponsor. The TA was received as an off-white powder. The PCA solution was prepared to be 30% PCA w/v in Ethanol. The PCA was prepared in 5g increments to pre-warmed 50-60 mL ethanol until dissolved for a total of 30g PCA in the solution. Additional ethanol was then added volumetrically to be equivalent to 100mL.
The Test Substrates (TS) were a Plastic-type material sourced from a clear plastic laboratory bottle (Corning 431731 Octagonal bottle, 150mL), cloth (the top layer of a N95 mask [3M 8210]), and a Sponsor-provided wire mesh to serve as a substrate for the TA. All test substrates were cut to approximately 1”x 1” in size. The test substrates were submerged into the PCA solution and dried horizontally to allow for even coating. After the substrate was thoroughly dried, the test substrate was re-submerged into the PCA solution for an additional coating.
Test Virus and Cell Culture.
The Test Virus used for this study was 2019 Novel Coronavirus, Isolate USA-WA1/2020 (SARS-CoV-2). The virus was stored at approximately ≤ -65°C prior to use. The multiplicity of infection (MOI) was 0.01 TCID50/cell.
The Cell Culture used for the TCID50 test was African Green Monkey Kidney Cells (Vero E6 cells) that were maintained in Dulbecco’s Minimum Essential Medium with 10% fetal calf serum (DMEM-2). All growth media contained heat-inactivated fetal calf serum and antibiotics.
Study Design:
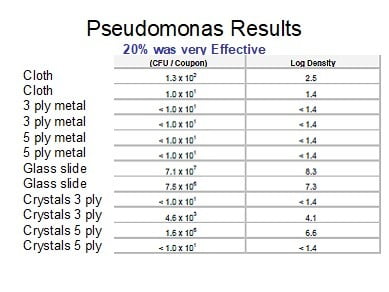
The test design is shown below in Table 1. This test will assess the TA on a substrate in various conditions as shown in Table 1.
- The Test Substrates were coated with PCA as described above. The test substrates were treated with PCA twice and allowed to fully dry overnight. In general, the time from the first coat to the next day’s virus exposure was approximately 24 hours and the second coat was applied approximately 203 hours later. Total time between the final PCA coat to the initiation of the study was approximately 21-22 hours. The coated test substrates were stored in a Safety Hood that was continuously operating until the initiation of the study.
- The PCA-treated Test Substrate was placed into a sterile 6 well cell culture plate and approximately 100 µL total of a ≥1 x 106 TCID50/mL SARS-CoV-2 virus was such that 50 µL of virus was layered on each sides of the treated test substrates. This was the procedure used for the initial Day 1 experiment.
For the Day 2 test, in an attempt to increase the recorded titer of the controls, the treated test substrate plus TA was placed into a sterile 6 well cell culture plate and the same amount of virus was layered onto both sides of the test substrate. However, an addition 50 µL of DMEM was added to each side to reduce the inactivation of the virus due to desiccation. Additionally, a glass coverslip was also added to help mitigate against evaporation. The Day 2 test also served as a confirmatory for the Day 1 test.
Note: In comparison between the Day 1 test to the Day 2 test procedures, the Day 1 test, examined the inactivation of the virus in an environment with less humidity/moisture than the Day 2 confirmatory test which was modified to have 100 µL (50 µL per side) extra DMEM and a cover slip to mitigate against evaporation.
- After application of the virus, the virus was contact with the test substrates for approximately 10 minutes (Groups 1, 2, and 3, Control groups 7, 8, and 9), 60 minutes (Groups 4, 5, and 6, Control Groups 10, 11, and 12). Each substrate per time per test article was performed in duplicate.
A cell culture-only control was included to indicate that cells without any TA or virus remain healthy throughout the assay. Virus-only controls without substrate was added for each time point to verify that the assay was performing as expected.
- After the incubation time, the treated substrates and control substrates were washed with 1 mL of cell culture media (DMEM-2) for approximately 5-10 minutes within the 6-well cell culture plate and the glass cover slip removed if necessary. This was the equivalent to a 10-fold dilution. The plate was gently stirred via an orbital shaker to enhance the recovery of the virus.
- For the TCID50, the cell culture media (DMEM-2) used to wash the test substrate was serially diluted 10 fold and transferred into respective wells of a 96-well plate which contained a monolayer of African Green Monkey Kidney Cells (Vero E6 cells) for titration. The TCID50 assay was performed non-GLP according to IITRI Standard Operating Procedures for the assay. The TCID50 titers were calculated using the method of Reed-Meunch.